[ad_1]
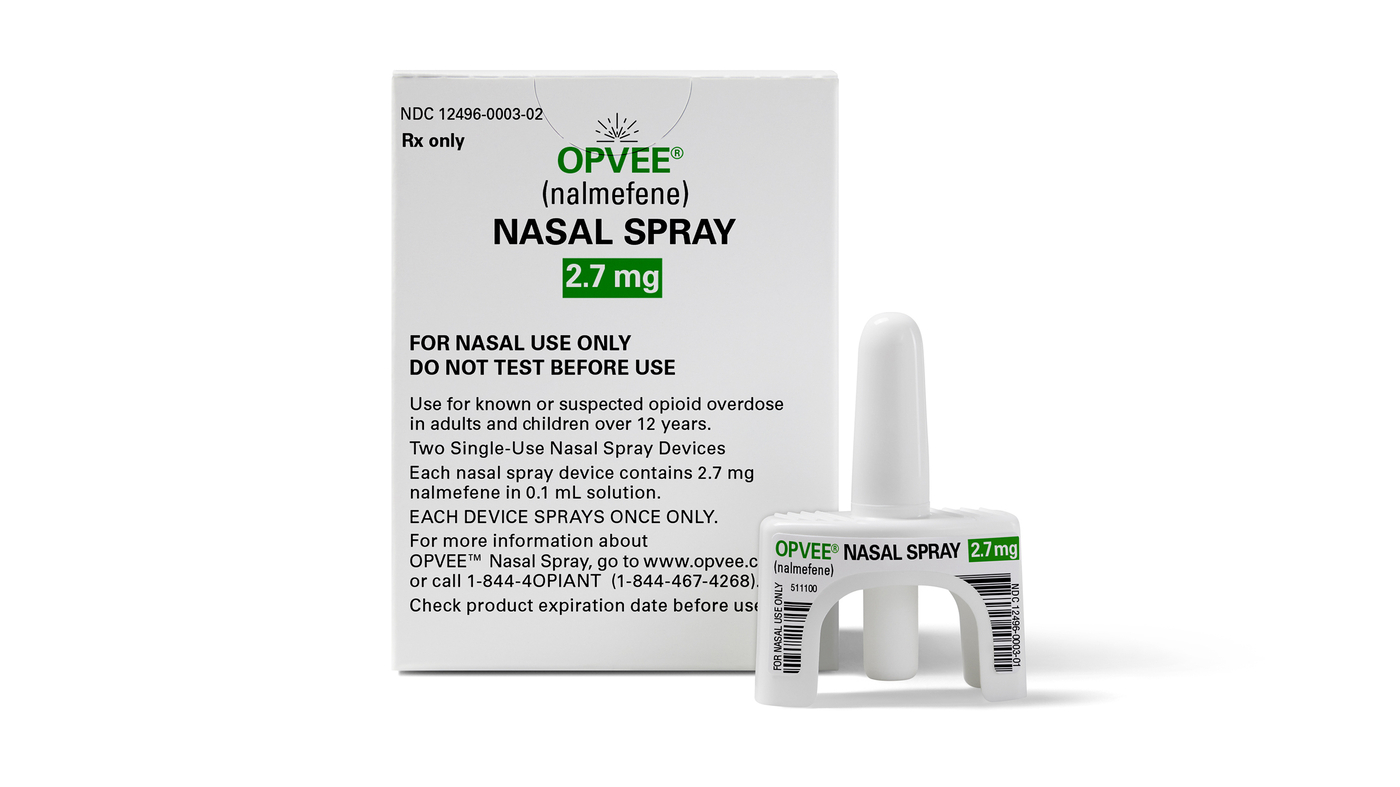
This photo provided by Indivior in May 2023 reveals their drug Opvee. U.S. health regulators accepted the medicine to reverse overdoses brought on by fentanyl and other potent opioids.
Indivior by way of AP
conceal caption
toggle caption
Indivior by way of AP

This photograph supplied by Indivior in Could 2023 demonstrates their drug Opvee. U.S. health regulators permitted the medication to reverse overdoses caused by fentanyl and other impressive opioids.
Indivior through AP
WASHINGTON — U.S. well being regulators on Monday approved a new quick-to-use model of a treatment to reverse overdoses prompted by fentanyl and other opioids driving the nation’s drug crisis.
Opvee is identical to naloxone, the everyday living-preserving drug that has been utilized for a long time to quickly counter overdoses of heroin, fentanyl and prescription painkillers. Both of those get the job done by blocking the effects of opioids in the mind, which can restore ordinary breathing and blood tension in people today who have lately overdosed.
The Foods and Drug Administration endorsed Opvee, a nasal spray update of the drug nalmefene, which was to start with approved as an injection in the mid-1990s but later eliminated from the market place due to minimal income. Naloxone will come as both equally a nasal spray and injection.
It truly is not right away distinct how the new drug will be applied in another way as opposed to naloxone, and some specialists see potential downsides to its lengthier-performing impact. The drug will be offered through prescription and is accepted for individuals 12 and more mature.
In scientific tests funded by the federal federal government, Opvee obtained related restoration outcomes to Narcan, the leading brand name of naloxone nasal spray.
Opvee was made by Opiant Prescription drugs, which was recently acquired by rival Indivior, maker of several remedies for opioid addiction. Indivior expects to launch Opvee in Oct at the earliest.
As the opioid epidemic has shifted to fentanyl and other synthetic opioids, researchers in the pharmaceutical market and the U.S. federal government noticed a new role for the drug.
Due to the fact fentanyl stays in the body for a longer time than heroin and other opioids, some individuals may well involve multiple doses of naloxone around numerous several hours to totally reverse an overdose.
Experts at the Nationwide Institutes of Wellness worked with pharmaceutical researchers on a nasal spray edition of nalmefene that would immediately resuscitate users, while also protecting them from relapse. Testing and development was funded by extra than $18 million in grants from the U.S. government’s Biomedical Advanced Analysis and Progress Authority and the NIH, which also assisted style and design the research.
“The entire aim of this was to have a treatment that would final extended but also attain into the mind really promptly,” said Dr. Nora Volkow, director of the Countrywide Institute on Drug Abuse.
Even now, some authorities see prospective downsides.
A side effect of all opioid reversal medicines is that they produce rigorous withdrawal indications which includes nausea, diarrhea, muscle cramps and panic. With naloxone, all those signs may final 30 to 40 minutes.
Dr. Lewis Nelson of Rutgers University says all those problems can very last 6 hours or extra with nalmefene, necessitating more treatment method and management by wellbeing pros.
“The risk of lengthy-lasting withdrawal is very serious and we try out to avoid it,” explained Nelson, an crisis medicine physician and previous adviser to the Fda on opioids.
Nelson claimed it really is quick plenty of to give a second or 3rd dose of naloxone if it wears off.
“We’re not struggling from a naloxone shortage in which we require to use an option,” he reported. “We have a lot of it and it is effective beautifully very well.”
The Fda approval will come as drug overdose deaths inched up a bit previous year soon after two big leaps for the duration of the pandemic. Much more than 109,000 deadly overdoses had been recorded in 2022, in accordance to the most up-to-date figures from the Centers for Disorder Handle and Prevention.
A lot more than two-thirds of people deaths were being joined to fentanyl and other synthetic opioids, which have mainly changed heroin and prescription opioids.
Naloxone has lengthy been at the middle of governing administration efforts to struggle the overdose crisis at the federal and nearby ranges. Police, firefighters and other initial responders routinely have the drug. And officials in all 50 states have provided orders to pharmacists to promote or dispense the drug devoid of a prescription to anybody who desires it.
In the most up-to-date federal drive, the Fda just lately approved Narcan to be bought in excess of the counter. The transform will make it possible for the new variation of the drug to be stocked in grocery outlets, vending machines and other retail destinations. The nasal spray — which involves up to date directions for normal end users — is predicted to launch this summer season. Emergent Biosolutions has not yet declared a price for the about-the-counter model.
Indivior mentioned it is nevertheless thinking of what to charge for its drug. It will compete in the very same marketplace as naloxone, in which most customers are local governments and local community teams that distribute to 1st responders and all those at chance of overdose. Indivior has informed buyers that Opvee could eventually make annual gross sales concerning $150 million to $250 million.
[ad_2]
Source link